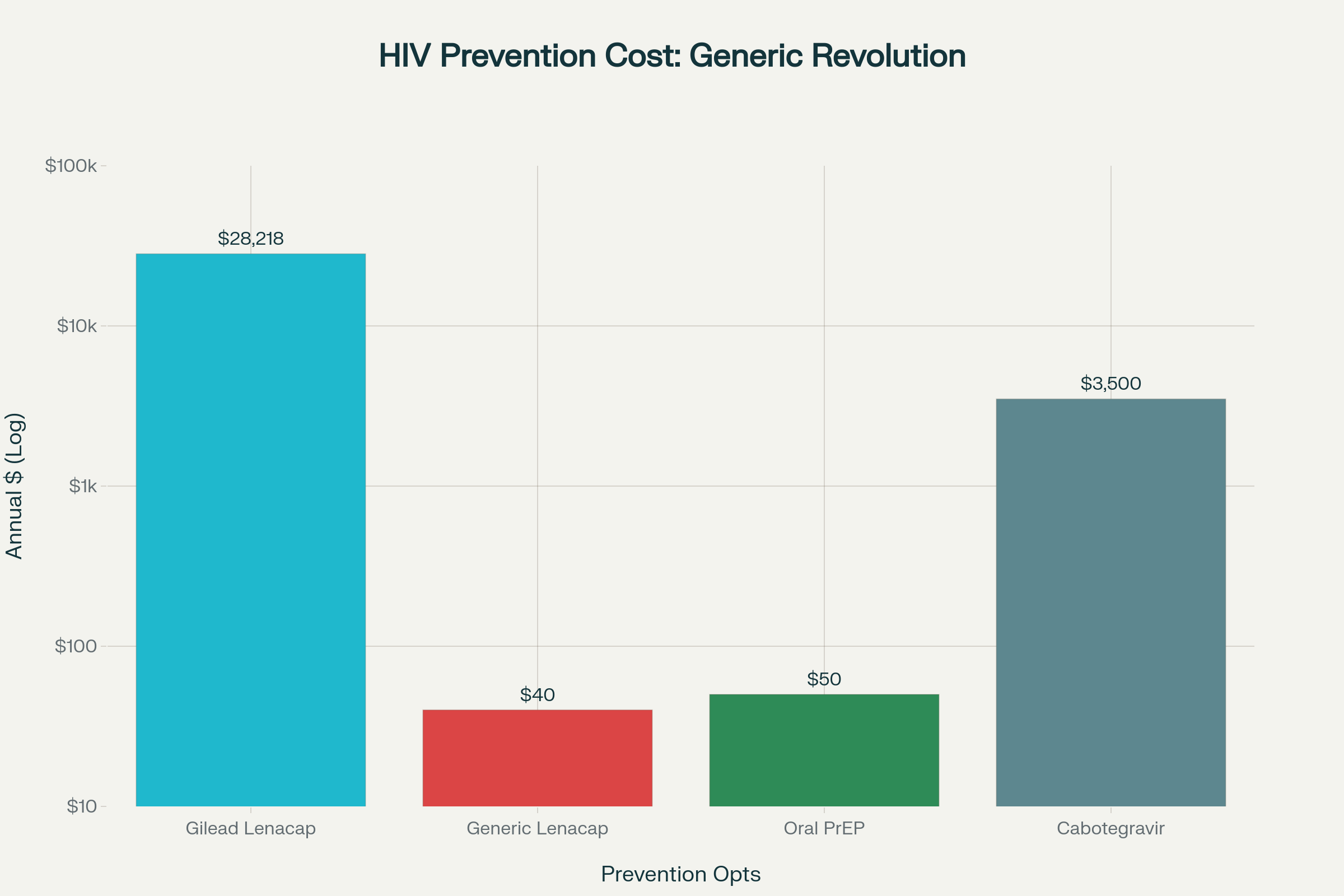
Indian pharmaceutical giants Dr. Reddy’s Laboratories and Hetero Labs have secured groundbreaking agreements to supply generic versions of the revolutionary lenacapavir HIV prevention drug at just $40 per year, representing a dramatic 700-fold price reduction from the original $28,218 annual cost. This landmark development positions the twice-yearly injectable lenacapavir HIV prevention tool to reach millions in 120 low- and middle-income countries starting 2027, potentially transforming global HIV prevention efforts.
Key Highlights
- Generic lenacapavir HIV prevention medication will be available at $40 annually in 120 countries through separate agreements with Dr. Reddy’s and Hetero Labs, supported by Unitaid, CHAI, Gates Foundation, and Wits RHI
- The drug demonstrated 99.9% effectiveness in preventing HIV infections in PURPOSE clinical trials, requiring only two injections per year
- Over 1.3 million people acquired HIV globally in 2024, with current prevention methods reaching only 18% of those who could benefit
Global HIV Prevention Landscape Transformation
The introduction of affordable generic lenacapavir HIV prevention addresses a critical gap in HIV prevention accessibility, particularly for populations struggling with daily oral medication adherence. Current global statistics reveal the urgent need for improved lenacapavir HIV prevention tools, with 40.8 million people living with HIV worldwide and 1.3 million new infections occurring annually despite existing prevention methods. The World Health Organization issued its recommendation for lenacapavir HIV prevention in July 2025, recognizing its potential to reshape HIV prevention strategies globally.
The drug’s twice-yearly injection schedule offers significant advantages over existing options, addressing barriers such as daily pill burden, frequent clinic visits, and stigma associated with HIV prevention. Research indicates that extending affordable lenacapavir HIV prevention to just 4% of people in high-burden countries could prevent up to 20% of new infections. This breakthrough comes at a critical time when HIV prevention efforts have stalled, with new infections remaining virtually unchanged from previous years.
At #CGI2025 today, President @BillClinton announced a landmark price agreement for lenacapavir: a twice-yearly HIV prevention injection will be available in low- and middle-income countries for US$40 per year.
— Unitaid (@UNITAID) September 24, 2025
Learn more: https://t.co/BtDv1rdAr1 pic.twitter.com/Od2NGmli5P
Unprecedented Manufacturing and Access Agreements
Dr. Reddy’s Laboratories has partnered with Unitaid, the Clinton Health Access Initiative, and Wits RHI to deliver quality-assured generic lenacapavir HIV prevention through comprehensive financial, technical, and regulatory support. The agreement positions Dr. Reddy’s among the first generic suppliers to enter the market, establishing competitive dynamics essential for long-term sustainability and supply security. The company will handle technology transfer, bioequivalence studies, product registration, and market launch across the designated 120 countries.
Separately, the Gates Foundation has committed over $80 million to support Hetero Labs through upfront funding and volume guarantees, ensuring manufacturing readiness and market preparation. Hetero’s agreement includes provisions for affordable active pharmaceutical ingredient supply, enabling other generic manufacturers to scale lenacapavir HIV prevention production efficiently. Both agreements were strategically negotiated to achieve price parity with current oral PrEP options, removing cost barriers that previously limited access in resource-constrained settings.
The foundation for these agreements was established in October 2024 when Gilead Sciences granted royalty-free licenses to six generic manufacturers, including Dr. Reddy’s and Hetero, covering production and distribution rights for 120 high-incidence, resource-limited countries. This collaborative approach between originator companies, generic manufacturers, and global health organizations represents a new model for accelerating access to breakthrough medical innovations.
Clinical Excellence and Regulatory Milestones
Lenacapavir HIV prevention exceptional clinical performance stems from landmark PURPOSE 1 and PURPOSE 2 trials that demonstrated unprecedented efficacy across diverse populations. The PURPOSE 2 trial showed a 96% reduction in HIV risk among participants, with 99.9% of individuals receiving lenacapavir HIV prevention remaining HIV-free compared to traditional daily oral prevention methods. Only two HIV infections occurred among 2,179 participants receiving lenacapavir HIV prevention, establishing it as the most effective HIV prevention tool currently available.
The drug’s regulatory journey achieved remarkable speed, with the U.S. Food and Drug Administration and European Medicines Agency providing approvals in record time. The World Health Organization followed with global recommendations in July 2025, acknowledging lenacapavir HIV prevention as a transformative addition to existing HIV prevention options including oral PrEP, dapivirine rings, and long-acting cabotegravir. This regulatory efficiency reflects the urgent global need for improved HIV prevention tools and the exceptional clinical evidence supporting lenacapavir HIV prevention safety and efficacy.
Science magazine recognized lenacapavir HIV prevention as its “2024 Breakthrough of the Year,” citing its astonishing 100% efficacy in preventing HIV infections among women in the PURPOSE 1 trial. This recognition underscores the drug’s potential to accelerate progress toward UNAIDS 2030 targets and fundamentally alter the trajectory of the global HIV epidemic.
Market Impact and Future Accessibility
The generic agreements create a competitive marketplace essential for sustained affordability and supply security across target countries. Multiple suppliers ensure price stability, prevent supply disruptions, and provide procurement flexibility for national health systems implementing lenacapavir HIV prevention programs. The initial oral regimen required alongside the first injection will cost no more than $17, maintaining affordability for the complete treatment protocol.
Gilead Sciences has simultaneously established partnerships with the Global Fund to supply doses for up to two million people over three years at no profit, demonstrating commitment to equitable access. The company aims to complete regulatory submissions in 18 countries by end-2025, representing areas with the highest HIV burden and greatest need for improved lenacapavir HIV prevention tools. These parallel efforts create multiple pathways for lenacapavir HIV prevention access while generic manufacturing capacity develops.
The pricing breakthrough positions lenacapavir HIV prevention competitively with existing oral PrEP options while offering superior convenience and efficacy. Research published in The Lancet HIV indicates that at-scale production could potentially reduce costs further to $25 per person-year with high demand, making the medicine accessible even in the lowest-income settings. This cost structure enables national health systems to consider lenacapavir HIV prevention as a viable alternative to current prevention strategies.
Strategic Assessment
The collaboration between Indian generic manufacturers and global health organizations establishes a new paradigm for accelerating access to breakthrough medical innovations. By achieving price parity with existing prevention methods while offering dramatically superior efficacy and convenience, generic lenacapavir HIV prevention removes traditional barriers that have limited HIV prevention uptake in resource-constrained settings. The 2027 timeline represents unprecedented speed in bringing generic versions of innovative medicines to low- and middle-income countries, typically a process requiring a decade or longer. This achievement demonstrates the potential for strategic partnerships to transform global health outcomes when originator companies, generic manufacturers, funding organizations, and regulatory agencies align around shared access objectives.


