The arrest of Ranganathan Govindan, owner of Sresan Pharmaceuticals, marks a critical breakthrough in India’s deadliest pharmaceutical contamination scandal involving Coldrif cough syrup. The Tamil Nadu-based manufacturer’s toxic product has claimed at least 20 children’s lives across multiple states, exposing catastrophic failures in drug quality control and regulatory oversight. Laboratory testing revealed the contaminated batch contained 48.6% diethylene glycol, nearly 500 times the permissible limit of 0.1%.
Key Highlights
- Ranganathan Govindan arrested in Chennai after midnight operation by Madhya Pradesh police following deaths of 20 children
- Coldrif syrup contained 48.6% diethylene glycol, a deadly industrial solvent used in antifreeze production
- Sresan Pharmaceuticals operated for 14 years with 364 documented violations including rusty equipment and contaminated facilities
Midnight Arrest Operation Brings Justice for Families
The dramatic arrest of Ranganathan Govindan concluded a coordinated manhunt across state lines after the Coldrif cough syrup manufacturer went into hiding following reports of children’s deaths. Madhya Pradesh police executed a meticulously planned midnight operation in Chennai, tracking the 73-year-old pharmaceutical owner’s vehicles and monitoring his residence before launching the arrest at 1:30 AM. The operation involved a specialized team of police officials and drug inspectors who had been stationed in Chennai since October 5, working with local authorities to apprehend the fugitive.
Following his arrest, authorities transported Govindan to his company’s Kancheepuram factory where they seized crucial documents related to the Coldrif cough syrup manufacturing process. The state government had announced a Rs 20,000 reward for his capture, reflecting the severity of charges including adulteration, culpable homicide not amounting to murder, and endangering children’s safety. Madhya Pradesh police are now seeking transit remand from a Chennai court to bring Ranganathan to Chhindwara, where the majority of deaths occurred.
The investigation will expand to cover the entire supply chain, including chemical suppliers, stockists, and medical representatives to identify every link in the deadly network that allowed the toxic Coldrif cough syrup to reach unsuspecting families. This comprehensive approach aims to prevent similar tragedies by addressing systemic failures in pharmaceutical distribution and quality control processes.
ऐसी जहरीली दवा बनाने वाली फार्मा कंपनियों पर हम रोक नहीं लगा पाएँगे! ऐसा करो
— Yashpal Arya (@IamYashpalArya) October 9, 2025
आप लोग खरीदो मत, पिओ मत।
ये ministry वाले क्या बेतुकी बाते करते है
फर्जी कंपनिया दवा के नाम पर जहर बना रही है
और भ्रष्ट और चंदा लेने वाला सिस्टम ही इन बच्चों की मौत का ज़िम्मेदार है।… pic.twitter.com/EYvQ9Fce4i
Devastating Impact Across Multiple States
The Coldrif cough syrup tragedy has resulted in 20 confirmed child deaths across multiple Indian states, with Madhya Pradesh bearing the heaviest toll. Chhindwara district alone recorded 17 fatalities, while Betul district reported 2 deaths and Pandhurna district witnessed 1 child’s death. Rajasthan has also reported deaths linked to the contaminated syrup, though specific numbers remain under investigation.
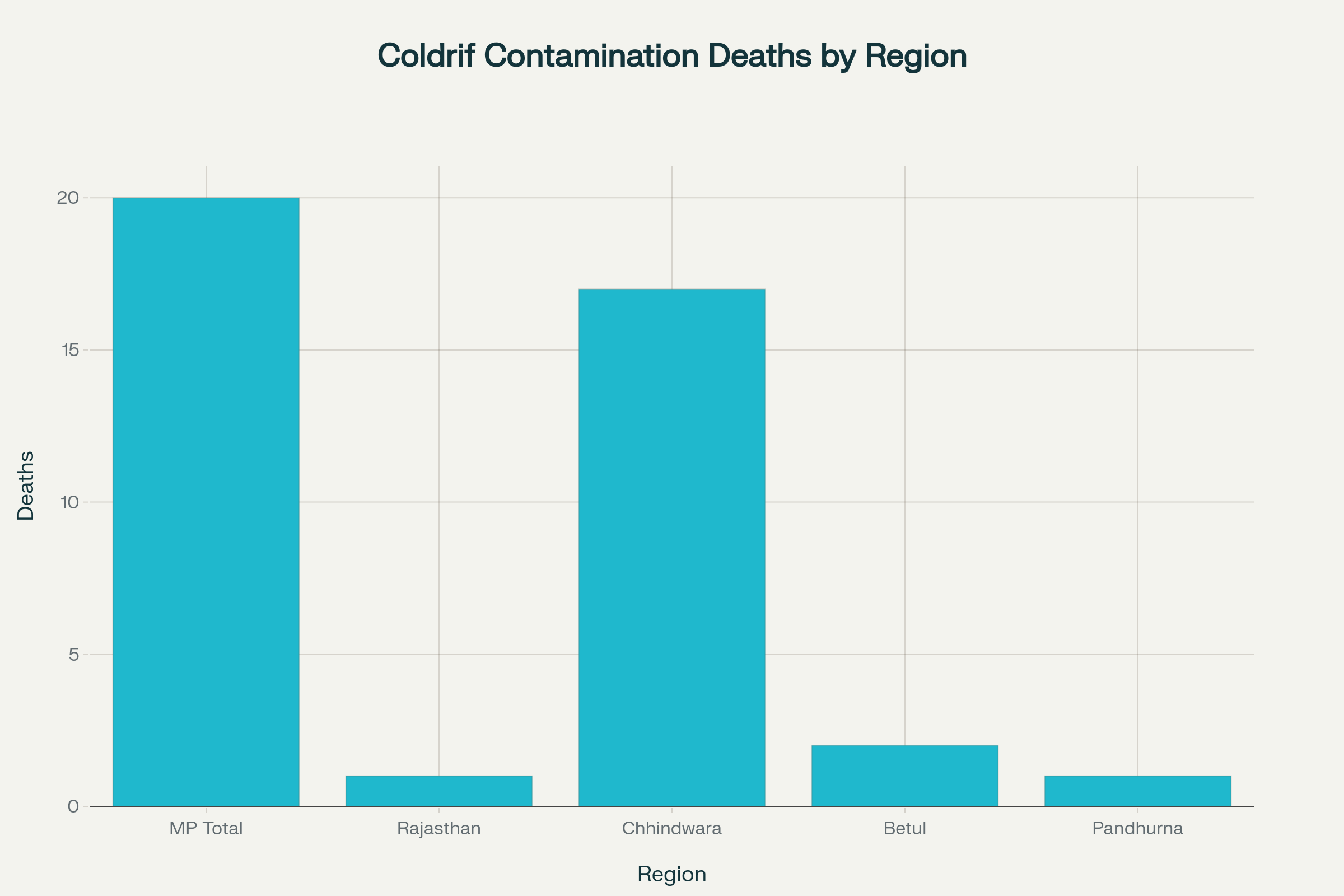
The victims, predominantly children under five years of age, developed acute kidney failure after consuming the contaminated Coldrif cough syrup prescribed for common cold and cough symptoms. The deaths began in late August 2025, when children in Chhindwara district started showing signs of sudden kidney failure after treatment for mild fevers and respiratory symptoms. Currently, five children remain hospitalized receiving treatment for complications arising from syrup consumption.
The Indian Council of Medical Research (ICMR) and Directorate General of Health Services (DGHS) have issued urgent advisories against prescribing cough syrups for children under four years old following this crisis. Medical experts emphasize that most childhood coughs are self-limiting and do not require pharmacological intervention, particularly given the significant risks associated with contaminated medications. At least nine states have banned the Coldrif cough syrup since the deaths were reported, reflecting the nationwide scope of concern.
Toxic Contamination Exposes Manufacturing Horrors
Laboratory analysis revealed that the contaminated Coldrif cough syrup contained 48.6% diethylene glycol (DEG), a highly toxic industrial solvent typically used in antifreeze, brake fluids, printing ink, and glue manufacturing. This concentration was approximately 480 times higher than the permissible limit of 0.1%, creating a lethal poison disguised as children’s medicine. Diethylene glycol causes severe kidney, liver, and nervous system damage in humans, with ingestion of even small quantities leading to acute kidney failure and death.
The Central Drugs Standard Control Organisation (CDSCO) confirmed that while samples collected from Madhya Pradesh tested negative for contamination, direct testing at Sresan Pharmaceuticals’ Tamil Nadu facility revealed the toxic contamination. The manufacturer used food-grade propylene glycol instead of pharmaceutical-grade propylene glycol as a solvent, violating fundamental drug manufacturing protocols. Food-grade propylene glycol requires removal of diethylene glycol impurities, a process that was clearly neglected at the Sresan facility.
Tamil Nadu’s drug control authorities discovered that Sresan Pharmaceuticals lacked essential testing equipment such as gas chromatography, which is crucial for detecting impurities in raw materials. The facility operated without proper quality control mechanisms, surveillance systems, or adequate testing protocols before and after manufacturing. This contaminated Coldrif cough syrup represents a catastrophic failure of basic pharmaceutical safety standards that should protect vulnerable pediatric patients.
Systemic Regulatory Failures Exposed
The Sresan Pharmaceuticals facility operated in what inspectors described as a “shoddy and neglected state” for 14 years without proper regulatory oversight. A central inspection team documented 364 total violations, including 39 critical and 325 major infractions that should have immediately triggered license cancellation. The facility lacked basic infrastructure including proper ventilation systems, purified water systems, pest control measures, and hygienic manufacturing practices.
Critical violations discovered at the Coldrif cough syrup manufacturing site included medicines stored in corridors, packaging operations conducted without air-handling units, and the use of rusty, cracked, and leaking equipment with high contamination risk. The company had no invoices or sourcing records for non-pharmaceutical grade propylene glycol, indicating potential procurement of substandard raw materials from unauthorized suppliers. Despite these extensive violations, Tamil Nadu’s drug authorities had never conducted a comprehensive audit of the facility since its licensing in 2011.
The regulatory response was deemed inadequate by central government officials, who questioned why state authorities issued only a stop-production order and show cause notice instead of immediately canceling the manufacturing license. This case highlights broader systemic issues within India’s pharmaceutical regulatory framework, where state-level oversight has proven insufficient to protect public health. The Coldrif cough syrup scandal has prompted India’s top drug regulator to admit serious lapses in medicine manufacturing practices, with advisories revealing that many factories fail to test every batch of raw materials and active ingredients.
Closing Assessment
The Coldrif cough syrup contamination represents one of India’s most devastating pharmaceutical disasters, claiming 20 young lives due to criminal negligence and regulatory failures. The arrest of Ranganathan Govindan provides some measure of justice for grieving families, but the broader systemic issues require comprehensive reform. This tragedy exposes the urgent need for strengthened oversight of India’s pharmaceutical sector, particularly small and medium enterprises that may lack adequate quality control systems.
The contaminated Coldrif cough syrup case serves as a stark reminder that regulatory vigilance cannot be compromised when children’s lives are at stake. Tamil Nadu’s health minister has confirmed that Sresan Pharmaceuticals’ license will be permanently revoked, while the investigation continues to expand across the entire supply chain. The implementation of stricter quality control measures, mandatory testing protocols, and enhanced regulatory enforcement represents the minimum response required to prevent future tragedies. This Coldrif cough syrup disaster must catalyze fundamental changes in pharmaceutical regulation to ensure that such preventable deaths never occur again.


